Abstract
Backgrounds:
Next-generation sequencing (NGS) based on liquid biopsy has been an emerging technology to identify tumor-specific genetic aberrations in adult lymphoma. However, there were few studies on the genomic profiling of plasma circulating tumor-derived DNA (ctDNA) in pediatric and adolescent lymphoblastic lymphoma (LBL).
Methods:
Paraffin-embedded tissue (FFPE), plasma and bone marrow samples from the newly diagnosed patients were collected and sequenced by 475 genes panel before, during and post of treatment. Clinical stage system, risk stratification and treatment of lymphoblastic lymphoma according to modified BFM-95 protocol.
Results:
A total of 68 patients (50 T-LBL, 18 B-LBL) from 13 centers in China were included in this study, including 49 male and 19 female, 3 patients in the low-risk group, 30 in the intermediate-risk group, and 19 in the high-risk group, and 16 patients were not stratified.
The baseline detection rate of tissue, plasma and bone marrow of the T-ALL/LBL patients were 96% (24/25), 97.14% (34/35), and 75% (12/16), respectively. The mean mutation abundance in baseline tissue (34.12%) was significantly higher than in plasma (15.16%) and bone marrow (13.93%).
Regarding sample consistency compared with tissue, the sensitivity of baseline plasma and bone marrow were 73.83% (79/107, N=18) and 70.77% (46/65, N=10), respectively. Compared with plasma, the sensitivity of mutation detection in bone marrow samples was 67.29% (72/107%, N=14). There were no significant correlation between ctDNA mutation abundance, concentration and risk stratification, stage, and SUV of PET/CT.
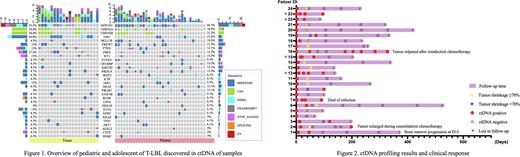
The most common molecular abnormality detected in tissue and plasma was NOTCH1 mutation (52.2% in tissue, 66.7% in plasma), and the mutation types were mainly missense mutation, deletion, and frameshift mutation, and also found rare NOTCH1 gene fusions (IKZF2: exon5~NOTCH1: exon28, NOTCH1: exon 30~IKZF2: exon6). Other high-frequency gene variants were CDKN2A (tissue 30.4%, plasma 18.2%), CDKN2B (tissue 30.4%, plasma 21.2%), JAK1 (tissue 34.8%, plasma 15.2%), BCL11B (tissue 4.3%, plasma 18.2%) FBXW7 (tissue 13%, plasma 21.2%), PTEN (tissue 13%, plasma 12.1%) and so on (Figure 1).
A total of 24 patients underwent dynamic monitoring of two or more liquid biopsies (Figure 2), and all patients were positive in baseline plasma ctDNA. At the last follow-up time, 11 of 24 patients were at least 2 times positive for ctDNA examination, of whom 5 patients with tumor recurrence, progression or poor prognosis (3 cases poor response to induction IA chemotherapy with tumor shrunk less than 70%, 2 cases with tumor progression and 1 case with tumor relapsed) and 1 patient died of infection during consolidation treatment and 4 cases lost to follow-up. Twelve of 13 patients whose plasma ctDNA cleared at the second examination time achieved PR with tumors shrunk more than 70% after induction IA treatment and none of the 13 patients relapsed or disease progression.
In B-LBL, the baseline detection rate of tissue, plasma and bone marrow were 75% (9/12), 100% (12/12), and 66.67% (4/6), respectively. The top five gene variants were CDKN2A (26.7%), FLT3 (26.7%), NRAS (26.7%), KRAS (20%), NTRK3 (20%).
Conclusion:
Plasma ctDNA testing by NGS was practicable in pediatric and adolescent lymphoblastic lymphoma and could well reflect the clinical efficacy and detect tumor recurrence. Nevertheless, it is necessary to expand the sample size and extend the follow-up time to explore the value of liquid biopsy in pediatric and adolescent lymphoblastic lymphoma.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal